Uranium occurs naturally in low concentrations in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. Uranium ore can be mined from open pits or underground excavations. The ore can then be crushed and treated at a mill to separate the valuable uranium from the ore. Uranium may also be dissolved directly from the ore deposits in the ground (in-situ leaching) and pumped to the surface. Uranium mined from the earth is stored, handled, and sold as uranium oxide concentrate (U3O8).
Uranium was discovered in 1789 by Martin Klaproth, a German chemist, who isolated an oxide of uranium while analyzing pitchblende samples from the Joachimsthal silver mines in the former Kingdom of Bohemia, located in present-day Czechia. He named his discovery “uran” after the planet Uranus.
For many years, uranium was used primarily as a colorant for ceramic glazes and for tinting in early photography. Its radioactive properties were not recognized until 1866, and its potential for use as an energy source was not manifested until the mid-20th century. Uranium is now used to power commercial nuclear reactors that produce electricity and to produce isotopes used for medical, industrial, and defense purposes around the world.
Physical Properties of Uranium
- Concentration - Uranium ranks 48th among the most abundant elements found in natural crustal rock. In the Earth’s crust, uranium is found as a mineral, bonded with other elements.
- Density - Uranium metal is very dense. At about 19 grams per cubic centimeter, it is 1.67 times more dense than lead.
Isotopic Properties of Uranium
- Natural Uranium – contains a 238U concentration of 99.27 percent, 235U concentration of 0.711 percent and very little 234U.
- Low Enriched Uranium – contains a 235U concentration between 0.711 percent and 20 percent. Most commercial reactor fuel uses low enriched uranium (LEU) enriched to between 3 percent and 5 percent 235U. Uranium between 3 and 5 percent 235U is sometimes referred to as “reactor-grade uranium.”
- Highly Enriched Uranium – contains a 235U concentration greater than 20 percent. Highly enriched uranium (HEU) is used in naval propulsion reactors, nuclear weapons and in some research reactors.
- Depleted Uranium – contains a 235U concentration of 0.711 percent or less. It is a co-product of the enrichment process.
What is Uranium? How Does it Work?
- Uranium is a heavy metal which has been used as an abundant source of concentrated energy for over 60 years.
- Uranium occurs in most rocks in concentrations of 2 to 4 parts per million and is as common in the Earth's crust as tin, tungsten and molybdenum. Uranium occurs in seawater, and can be recovered from the oceans.
- Uranium was discovered in 1789 by Martin Klaproth, a German chemist, in the mineral called pitchblende. It was named after the planet Uranus, which had been discovered eight years earlier.
- Uranium was apparently formed in supernovae about 6.6 billion years ago. While it is not common in the solar system, today its slow radioactive decay provides the main source of heat inside the Earth, causing convection and continental drift.
- The high density of uranium means that it also finds uses in the keels of yachts and as counterweights for aircraft control surfaces, as well as for radiation shielding.
- Uranium has a melting point of 1132°C. The chemical symbol for uranium is U.
The uranium atom
On a scale arranged according to the increasing mass of their nuclei, uranium is one of the heaviest of all the naturally-occurring elements (hydrogen is the lightest). Uranium is 18.7 times as dense as water.
Like other elements, uranium occurs in several slightly differing forms known as 'isotopes'. These isotopes differ from each other in the number of uncharged particles (neutrons) in the nucleus. Natural uranium as found in the Earth's crust is a mixture largely of two isotopes: uranium-238 (U-238), accounting for 99.3% and uranium-235 (U-235) about 0.7%.
The isotope U-235 is important because under certain conditions it can readily be split, yielding a lot of energy. It is therefore said to be 'fissile' and we use the expression 'nuclear fission'.
Meanwhile, like all radioactive isotopes, they decay. U-238 decays very slowly, its half-life being about the same as the age of the Earth (4500 million years). This means that it is barely radioactive, less so than many other isotopes in rocks and sand. Nevertheless it generates 0.1 watts/tonne as decay heat and this is enough to warm the Earth's core. U-235 decays slightly faster.
Energy from the uranium atom
The nucleus of the U-235 atom comprises 92 protons and 143 neutrons (92 + 143 = 235). When the nucleus of a U-235 atom captures a moving neutron it splits in two (fissions) and releases some energy in the form of heat, also two or three additional neutrons are thrown off. If enough of these expelled neutrons cause the nuclei of other U-235 atoms to split, releasing further neutrons, a fission 'chain reaction' can be achieved. When this happens over and over again, many millions of times, a very large amount of heat is produced from a relatively small amount of uranium.
It is this process, in effect 'burning' uranium, which occurs in a nuclear reactor. The heat is used to make steam to produce electricity.
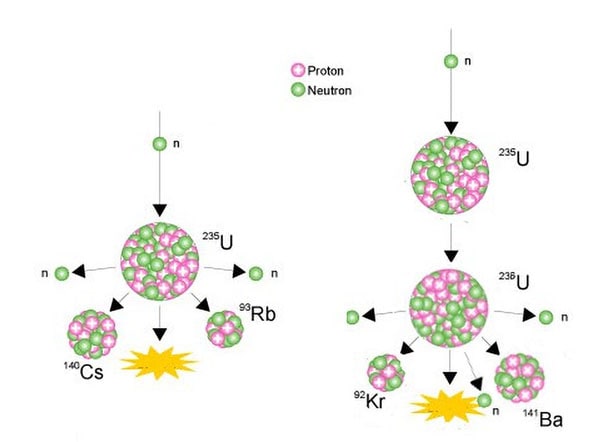
Examples of nuclear fissioning of uranium-235
Inside the reactor
Nuclear power stations and fossil-fuelled power stations of similar capacity have many features in common. Both require heat to produce steam to drive turbines and generators. In a nuclear power station, however, the fissioning of uranium atoms replaces the burning of coal or gas. In a nuclear reactor the uranium fuel is assembled in such a way that a controlled fission chain reaction can be achieved. The heat created by splitting the U-235 atoms is then used to make steam which spins a turbine to drive a generator, producing electricity.
The chain reaction that takes place in the core of a nuclear reactor is controlled by rods which absorb neutrons and which can be inserted or withdrawn to set the reactor at the required power level.
The fuel elements are surrounded by a substance called a moderator to slow the speed of the emitted neutrons and thus enable the chain reaction to continue. Water, graphite and heavy water are used as moderators in different types of reactor.
Because of the kind of fuel used (i.e. the concentration of U-235, see below), if there is a major uncorrected malfunction in a reactor the fuel may overheat and melt, but it cannot explode like a bomb.
A typical 1000 megawatt (MWe) reactor can provide enough electricity for a modern city of up to one million people.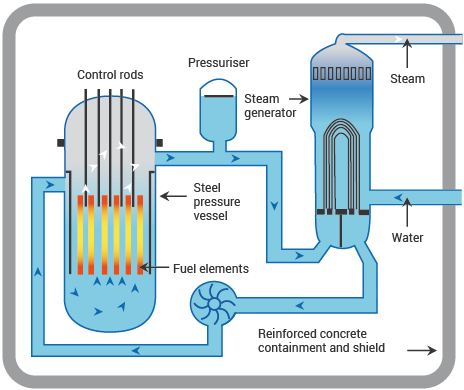
Uranium and plutonium
Whereas the U-235 nucleus is 'fissile', that of U-238 is said to be 'fertile'. This means that it can capture one of the neutrons which are flying about in the core of the reactor and become (indirectly) plutonium-239, which is fissile. Pu-239 is very much like U-235, in that it fissions when hit by a neutron and this yields a similar amount of energy.
Because there is so much U-238 in a reactor core (most of the fuel), these reactions occur frequently, and in fact about one-third of the fuel's energy yield comes from 'burning' Pu-239.
But sometimes a Pu-239 atom simply captures a neutron without splitting, and it becomes Pu-240. Because the Pu-239 is either progressively 'burned' or becomes Pu-240, the longer the fuel stays in the reactor the more Pu-240 is in it. (The significance of this is that when the spent fuel is removed after about three years, the plutonium in it is not suitable for making weapons but can be recycled as fuel.)
From uranium ore to reactor fuel
Uranium ore can be mined by underground or open-cut methods, depending on its depth. After mining, the ore is crushed and ground up. Then it is treated with acid to dissolve the uranium, which is recovered from solution.
Uranium may also be mined by in situ leaching (ISL), where it is dissolved from a porous underground ore body in situ and pumped to the surface.
The end product of the mining and milling stages, or of ISL, is uranium oxide concentrate (U3O8). This is the form in which uranium is sold.
Before it can be used in a reactor for electricity generation, however, it must undergo a series of processes to produce a useable fuel.
For most of the world's reactors, the next step in making the fuel is to convert the uranium oxide into a gas, uranium hexafluoride (UF6), which enables it to be enriched. Enrichment increases the proportion of the uranium-235 isotope from its natural level of 0.7% to 4-5%. This enables greater technical efficiency in reactor design and operation, particularly in larger reactors, and allows the use of ordinary water as a moderator.
After enrichment, the UF6 gas is converted to uranium dioxide (UO2) which is formed into fuel pellets. These fuel pellets are placed inside thin metal tubes, known as fuel rods, which are assembled in bundles to become the fuel elements or assemblies for the core of the reactor. In a typical large power reactor there might be 51,000 fuel rods with over 18 million pellets.

A worker holds up a newly made fuel pellet (KazAtomProm)
For reactors which use natural uranium as their fuel (and hence which require graphite or heavy water as a moderator) the U3O8 concentrate simply needs to be refined and converted directly to uranium dioxide.
When the uranium fuel has been in the reactor for about three years, the used fuel is removed, stored, and then either reprocessed or disposed of underground (see Nuclear Fuel Cycle or Radioactive Waste Management).
Who uses nuclear power?
About 10% of the world's electricity is generated from uranium in nuclear reactors. This amounts to over 2500 TWh each year, as much as from all sources of electricity worldwide in 1960.
It comes from about 440 nuclear reactors with a total output capacity of about 390,000 megawatts (MWe) operating in 32 countries. About 60 more reactors are under construction and about 100 are planned.
Belgium, Bulgaria, Czech Republic, Finland, France, Hungary, Slovakia, Slovenia, Sweden and Ukraine all get 30% or more of their electricity from nuclear reactors. The USA has about 90 reactors operating, supplying 20% of its electricity. France gets about 70% of its electricity from uranium.
Over the 60 years that the world has enjoyed the benefits of cleanly-generated electricity from nuclear power, there have been about 18,500 reactor-years of operational experience.
See also Nuclear Generation by Country.
Who has and who mines uranium?
Uranium is widespread in many rocks, and even in seawater. However, like other metals, it is seldom sufficiently concentrated to be economically recoverable. Where it is, we speak of an orebody. In defining what is ore, assumptions are made about the cost of mining and the market price of the metal. Uranium reserves are therefore calculated as tonnes recoverable up to a certain cost.
Uranium resources by country in 2021
tonnes U percentage of world Australia 1,684,10028%Kazakhstan 815,20013%Canada 588,50010%Russia 480,9008%Namibia 470,100 8% South Africa 320,9005%Niger 311,100 5% Brazil 276,800 5% China 223,900 4% Mongolia 144,600 2% Uzbekistan 131,3002%Ukraine 107,2002%Botswana 87,2001%Tanzania 58,2001%Jordan 52,500 1% USA 59,400 1% Other 266,6005%World total 6,078,500Identified resources recoverable (reasonably assured resources plus inferred resources), to $130/kg U, 1/1/21, from OECD NEA & IAEA, Uranium 2022: Resources, Production and Demand ('Red Book'). The total recoverable identified resources to $260/kg U is 7.918 million tonnes U.
Production from mines (tonnes U)
Country 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 Kazakhstan 22,451 23,127 23,607 24,689 23,321 21,705 22,808 19,477 21,819 21,227 Canada 9331 9134 13,325 14,039 13,116 7001 6938 3885 4693 7351 Namibia 4323 3255 2993 3654 4224 5525 5476 5413 5753 5613 Australia 6350 5001 5654 6315 5882 6517 6613 6203 4192 4087 Uzbekistan (est.) 2400 2400 2385 3325 3400 3450 3500 3500 3520 3300 Russia 3135 2990 3055 3004 2917 2904 2911 2846 2635 2508 Niger 4518 4057 4116 3479 3449 2911 2983 2991 2248 2020 China (est.) 1500 1500 1616 1616 1692 1885 1885 1885 1600 1700 Ukraine 922 926 1200 808 707 790 800 744 455 100 India (est.) 385 385 385 385 421 423 308 400 600 600 South Africa (est.) 531 573 393 490 308 346 346 250 192 200 Iran (est.) 0 0 38 0 40 71 71 71 21 20 Pakistan (est.) 45 45 45 45 45 45 45 45 45 45 USA 1792 1919 1256 1125 940 582 58 6 8 75 Brazil 192 55 40 44 0 0 0 15 29 43 Czech Republic 215 193 155 138 0 0 0 0 0 0 Romania 77 77 77 50 0 0 0 0 0 0 France 5 3 2 0 0 0 0 0 0 0 Germany 27 33 0 0 0 0 0 0 0 0 Malawi 1132 369 0 0 0 0 0 0 0 0 Total world 59,331 56,041 60,304 63,207 60,514 54,154 54,742 47,731 47,808 48,888 tonnes U3O8 69,966 66,087 71,113 74,357 71,361 63,861 64,554 56,287 56,377 57,651 % of world demand 91% 85% 98% 96% 93% 80% 81% 74% 76% 74% * Data from the World Nuclear Association. NB: the figures in this table are liable to change as new data becomes available. Totals may not sum exactly due to rounding.
Mining methods have been changing. In 1990, 55% of world production came from underground mines, but this shrunk dramatically to 1999, with 33% then. From 2000 the new Canadian mines increased it again. In situ leach (ISL, also called in situ recovery, ISR) mining has been steadily increasing its share of the total, mainly due to Kazakhstan, and in 2022 accounted for over 55% of production:
Method tonnes U % In situ leach (ISL) 27,307 56% Underground & open pit (except Olympic Dam) 18,569 38% By-product 3013 6% Other uses of nuclear energy
Uranium is sold only to countries which are signatories of the Nuclear Non-Proliferation Treaty (NPT), and which allow international inspection to verify that it is used only for peaceful purposes.
Many people, when talking about nuclear energy, have only nuclear reactors (or perhaps nuclear weapons) in mind. Few people realise the extent to which the use of radioisotopes has changed our lives over the last few decades.
Using relatively small special-purpose nuclear reactors, it is possible to make a wide range of radioactive materials (radioisotopes) at low cost. For this reason the use of artificially-produced radioisotopes has become widespread since the early 1950s, and there are now about 220 'research' reactors in 56 countries producing them. These are essentially neutron factories rather than sources of heat.
Radioisotopes
In our daily life we need food, water and good health. Today, radioactive isotopes play an important part in the technologies that provide us with all three. They are produced by bombarding small amounts of particular elements with neutrons.
In medicine, radioisotopes are widely used for diagnosis and research. Radioactive chemical tracers emit gamma radiation which provides diagnostic information about a person's anatomy and the functioning of specific organs. Radiotherapy also employs radioisotopes in the treatment of some illnesses, such as cancer. About one person in two in the Western world is likely to experience the benefits of nuclear medicine in their lifetime. More powerful gamma sources are used to sterilise syringes, bandages and other medical utensils – gamma sterilisation of equipment is almost universal.
In the preservation of food, radioisotopes are used to inhibit the sprouting of root crops after harvesting, to kill parasites and pests, and to control the ripening of stored fruit and vegetables. Irradiated foodstuffs are accepted by world and national health authorities for human consumption in an increasing number of countries. They include potatoes, onions, dried and fresh fruits, grain and grain products, poultry and some fish. Some prepacked foods can also be irradiated.
In the growing of crops and breeding livestock, radioisotopes also play an important role. They are used to produce high yielding, disease-resistant and weather-resistant varieties of crops, to study how fertilisers and insecticides work, and to improve the productivity and health of domestic animals.
Industrially, and in mining, they are used to examine welds, to detect leaks, to study the rate of wear of metals, and for on-stream analysis of a wide range of minerals and fuels.
There are many other uses. A radioisotope derived from the plutonium formed in nuclear reactors is used in most household smoke detectors.
Radioisotopes are used to detect and analyse pollutants in the environment, and to study the movement of surface water in streams and also of groundwater.
See also The Many Uses of Nuclear Technology.
Other reactors
There are also other uses for nuclear reactors. About 200 small nuclear reactors power some 150 ships, mostly submarines, but ranging from icebreakers to aircraft carriers. These can stay at sea for long periods without having to make refuelling stops. In the Russian Arctic where operating conditions are beyond the capability of conventional icebreakers, very powerful nuclear-powered vessels operate year-round, where previously only two months allowed northern access each year.
The heat produced by nuclear reactors can also be used directly rather than for generating electricity. In Sweden, Russia and China, for example, surplus heat is used to heat buildings. Nuclear heat may also be used for a variety of industrial processes such as water desalination. Nuclear desalination is likely to be a major growth area in the next decade.
High-temperature heat from nuclear reactors is likely to be employed in some industrial processes in future, especially for making hydrogen.
Military sources of fuel
Both uranium and plutonium were used to make bombs before they became important for making electricity and radioisotopes. The type of uranium and plutonium for bombs is different from that in a nuclear power plant. Bomb-grade uranium is highly-enriched (>90% U-235, instead of up to 5%); bomb-grade plutonium is fairly pure Pu-239 (>90%, instead of about 60% in reactor-grade) and is made in special reactors.
Since the 1990s, due to disarmament, a lot of military uranium has become available for electricity production. The military uranium is diluted about 25:1 with depleted uranium (mostly U-238) from the enrichment process before being used in power generation. Over two decades to 2013 one-tenth of US electricity was made from Russian weapons uranium.

Uranium(VI) oxides or "yellow cake" is an intermediate step in the processing of uranium ores.
(courtesy of www.chemcases.com)Uranium
Atomic Number: 92 Atomic Radius: 240 pm (Van der Waals) Atomic Symbol: U Melting Point: 1133 °C Atomic Weight: 238 Boiling Point: 4131 °C Electron Configuration: [Rn]7s25f36d1 Oxidation States: 6, 5, 4, 3,[2] 2, 1 History
The use of uranium in its natural oxide form dates back to 79 A.D. when it was used as a yellow coloring agent in ceramic glazes. Yellow glass with 1% uranium oxide was found in an ancient Roman villa near Naples, Italy. In the late Middle Ages, pitchblende was extracted from the silver mines and was used as a coloring agent in the glassmaking industry. The identification of uranium as an element is generally credited to Martin H. Klaproth. While experimenting with pitchblende in 1789, he concluded that it contained a new element, which he named after the newly discovered planet Uranus (named after the Greek god of the sky or heaven). What Klaproth actually identified was not the pure element but uranium oxide. The pure metal was first isolated in 1841 by Eugène-Melchior Péligot, who reduced anhydrous uranium tetrachloride with potassium metal.
In 1896 Antoine H. Becquerel discovered that uranium exhibited invisible light or rays; it was radioactivity. In 1934 research by Enrico Fermi and others eventually led to the use of uranium fission in the first nuclear weapon used in war and later in the peaceful use of uranium as fuel in nuclear power production. An ensuing arms race during the Cold War between the United States and the Soviet Union produced tens of thousands of nuclear weapons that used uranium metal and uranium-derived plutonium-239. The security of those weapons and their fissile material following the breakup of the Soviet Union in 1991 is an ongoing concern.
In 1972 French physicist Francis Perrin discovered ancient and no longer active prehistoric natural nuclear fission reactors in uranium ore deposits at the Oklo mine in Gabon, West Africa, collectively known as the Oklo Fossil Reactors. The ore deposit is 1.7 billion years old; at that time, uranium-235 constituted about 3% of the total uranium on Earth (0.72% today). This is high enough to permit a sustained nuclear fission chain reaction to occur, provided other supporting geologic conditions exist.
Isotopes
Uranium is weakly radioactive because all naturally occurring (or primordial) isotopes of uranium (238U, 235U and 234U) are unstable, with half-lives varying between 159,200 years and 4.5 billion years. There are 27 known isotopes of uranium ranging in atomic weights 217–219, 222–240 and 242, with half-lives of from billions of years to a few nanoseconds. Naturally occurring uranium consists of three major isotopes: 238U (99.28% abundance), 235U (0.71%), and 234U (0.0054%). (The US DOE has adopted the value of 0.711 as being their official percentage of 235U in natural uranium.) All three isotopes are radioactive, with small probabilities of undergoing spontaneous fission but preferentially decaying by alpha emission. The half-life of uranium-238 is about 4.47 billion years and that of uranium-235 is 704 million years, making them useful in dating the age of the Earth. It also suggests that half of the uranium that existed from the formation of the Earth has decayed to other radioactive elements and eventually to stable elements. Much of the internal heat of the earth is thought to be attributable to the decay of uranium and thorium radio-isotopes.
Uranium-238 is an α-particle emitter (occasionally, it undergoes spontaneous fission), decaying through the "Uranium Series" of nuclear decay, which has 18 members, all of which eventually decay into lead-206, by a variety of different decay paths. The decay series of 235U, which is called the actinium series has 15 members, all of which eventually decay into lead-207. The constant rates of decay in these decay series make the comparison of the ratios of parent to daughter elements useful in radiometric dating. Uranium-234 is a member of the "Uranium Series", and it decays to lead-206 through a series of relatively short-lived isotopes. Uranium-233 is made from thorium-232 by neutron bombardment, usually in a nuclear reactor, and 233U is also fissile. Its decay series ends with thallium-205.
Sources
Uranium is the heaviest naturally-occurring element available in large quantities. The heavier “transuranic” elements are either man-made or they exist only as trace quantities in uranium ore deposits as activation products. Uranium occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals. Uranium, not as rare as once thought, is now considered to be more plentiful than mercury, antimony, silver, or cadmium, and is about as abundant as molybdenum or arsenic. It occurs in numerous natural minerals such as pitchblende, uraninite, carnotite, autunite, uranophane, and tobernite. It is also found in phosphate rocks, lignite, monazite sands, and is recovered commercially from these sources. The United States Department of Energy purchases uranium in the form of acceptable U3O8 concentrates. This incentive program has greatly increased the known uranium reserves.
Properties
Pure uranium is a silvery white, weakly radioactive metal, which is harder than most elements. It is malleable, ductile, slightly paramagnetic, strongly electropositive and is a poor electrical conductor. Uranium metal has very high density, being approximately 70% denser than lead, but slightly less dense than gold. Uranium metal exhibits in three crystallographic modifications: alpha --> (688°C) --> beta --> (776°C) --> gamma. Uranium is pyrophoric when finely divided. It is a little softer than steel and is attacked by cold water in a finely divided state.In air, uranium metal becomes coated with a layer of oxide. Acids dissolve the metal, forming the +3 oxidation state which oxidizes rapidly by water and air to form higher oxidation states. Uranium metal is unaffected by alkalis. Uranium metal can be prepared by reducing uranium halides with alkali or alkaline earth metals or by reducing uranium oxides by calcium, aluminum, or carbon at high temperatures. The metal can also be produced by electrolysis of KUF5 or UF4, dissolved in a molten salt mixture of CaCl2 and NaCl. High-purity uranium can be prepared by the thermal decomposition of uranium halides on a hot filament.
Uranium metal reacts with almost all nonmetallic elements and their compounds, with reactivity increasing with temperature. Hydrochloric and nitric acids dissolve uranium, but non-oxidizing acids other than hydrochloric acid attack the element very slowly. When finely divided, it can react with cold water. In air, uranium metal oxidizes and becomes coated with a dark layer of uranium oxide. Uranium forms a variety of alloys and compounds with the most important oxidation states being uranium(IV) and uranium(VI), and their two corresponding oxides are, respectively, uranium dioxide, UO2 and uranium trioxide, UO3. Besides the oxides, other Important uranium compounds include fluorides, chlorides, bromides, iodides, carbonates, hydrides, carbides, nitrides, phosphates, etc. At room temperatures, uranium hexafluoride, UF6, has a high vapor pressure, making it useful in the gaseous diffusion process used to separate the rare U-235 from the common U-238 isotope. Uranium hydrides, nitrides and carbides are relatively inert semimetallic compounds that are minimally soluble in acids and have been used as stable fuel pellets in nuclear power reactor technology.
Uranium exists in aqueous solutions in the +3, +4, +5, and +6 oxidation states. Oxidation state +6 as the UO22+ ion (yellow in color) is the most stable state in solution. Uranium in the +5 state as the UO2+ ion is colorless, quite unstable and disproportionates (reacts with itself) to form the +6 and +4 states. The +4 state (green) is reasonably stable in solution, but the +3 state (dark green or dark red depending on the illumination source - daylight vs fluorescent light) is unstable and easily oxidizes to +4. The +4 state in near-neutral pH solutions readily hydrolyzes to form black oxy-hydroxide precipitates.
Uses
Uranium was used in as coloring agents in ceramic glazes and glass in ancient Rome and in the Middle Ages producing orange-red to lemon yellow hues. More recently it was used as an orange glaze in contemporary Fiestaware© dishware but was later discontinued for health reasons. Many contemporary uses of uranium exploit its unique nuclear properties. Uranium-235 has the distinction of being the only naturally occurring fissile isotope. This means it can be split into two or three fragments (fission products) by thermal neutrons. Uranium-238 is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239 in a nuclear reactor. Another fissile isotope, uranium-233, can be produced from natural thorium and is also important in nuclear technology. While uranium-238 has a small probability for spontaneous fission or even induced fission with fast neutrons, uranium-235 and to a lesser degree uranium-233 have a much higher fission cross-section for slow neutrons. In sufficient concentration, these isotopes maintain a sustained nuclear chain reaction. This generates the heat in nuclear power reactors, and produces the fissile material for nuclear weapons. This nuclear conversion can be brought about in breeder reactors where it is possible to produce more new fissionable material than the fissionable material used in maintaining the chain reaction. Depleted uranium (238U) (depleted of uranium-235) is used in balistic armor penetration and as armor plating.
Uranium-238 is not fissile, but is a fertile isotope, because after neutron activation it can produce plutonium-239, another fissile isotope. Indeed, the238U nucleus can absorb one neutron to produce the radioactive isotope uranium-239. 239U decays by beta emission to neptunium-239, also a beta-emitter, that decays in its turn, within a few days into plutonium-239. 239Pu was used as fissile material in the first atomic bomb detonated in the "Trinity test" on 15 July 1945 in New Mexico.
Uranium-235 is of even greater importance because it is the key to utilizing uranium. 235U, while occurring in natural uranium to the extent of only 0.71%, is so fissionable with slow neutrons that a self-sustaining fission chain reaction can be made in a reactor constructed from natural uranium and a suitable moderator, such as heavy water or graphite, alone.
Uranium-235 can be concentrated by gaseous diffusion and other physical processes, if desired, and used directly as a nuclear fuel, instead of natural uranium, or used as an explosive.
Natural uranium, slightly enriched with 235U by a small percentage, is used to fuel nuclear power reactors to generate electricity. Natural thorium can be irradiated with neutrons to produce the important isotope 233U as follows: 232Th(n, gamma) --> 233Th(beta) --> 233Pa(beta) --> 233U. While thorium itself is not fissionable, 233U is, and in this way may be used as a nuclear fuel. One pound of completely fissioned uranium has the fuel value of over 1500 tons of coal.
The uses of nuclear fuels to generate electrical power, to make isotopes for peaceful purposes, and to make explosives are well known. Uranium in the U.S.A. is controlled by the U.S. Nuclear Regulatory Commission. New uses are being found for depleted uranium, i.e., uranium with the percentage of 235U lowered to about 0.2%. Uranium is used in inertial guidance devices, in gyro compasses, as counterweights for aircraft control surfaces, as ballast for missile reentry vehicles, and as a shielding material. Uranium metal is used for X-ray targets for production of high-energy X-rays; the nitrate was once used as a photographic toner, and the acetate was once used in analytical chemistry. Crystals of uranium nitrate are triboluminescent. Uranium salts have also been used for producing yellow "Vaseline" glass and glazes.
Hazards
Uranium and its compounds are highly toxic, both from a chemical and radiological standpoint. Finely divided uranium metal, being pyrophoric, presents a fire hazard. In nature, U(VI) forms highly soluble carbonate complexes at alkaline pH. This leads to an increase in mobility and availability of uranium to groundwater and soil from nuclear waste repositories which leads to health hazards. Working with uranium requires the knowledge of the maximum allowable concentrations that may be inhaled or ingested. Recently, the natural presence of uranium in many soils has become of concern to homeowners because of the generation of radioactive radon gas and its daughters particularly in confined spaces with low circulation such as basements.







0 Comments